Blackmores Professional Duo Celloids® S.C.F.
Specific batches of the 170s & 84s containers
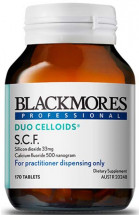
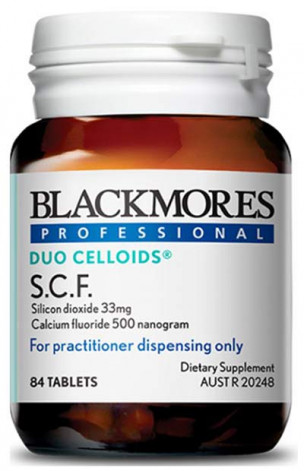
Product Identifiers
Potentially affected batches sold from January 2019 to June 2020 Blackmores Professional Duo Celloids® S.C.F. (170s and 84s) Batch Numbers: 292798, 294442, 295592, 296051, 297013, 298225, 298013 296052 No other batches or products are affected by this recall.Supplier Contact
If you have an adverse reaction, please seek medical attention and report your case to BioCeuticals customer -care support on 0508 006 823
Any adverse events should also be reported to the New Zealand Pharmacoviligance Centre’s Centre for Adverse Reactions Monitoring (CARM) at https://nzphvc.otago.ac.nz/consumer-reporting/.
The Hazard!
What to do...
Product Identifiers
Potentially affected batches sold from January 2019 to June 2020 Blackmores Professional Duo Celloids® S.C.F. (170s and 84s) Batch Numbers: 292798, 294442, 295592, 296051, 297013, 298225, 298013 296052 No other batches or products are affected by this recall.Supplier Contact
If you have an adverse reaction, please seek medical attention and report your case to BioCeuticals customer -care support on 0508 006 823
Any adverse events should also be reported to the New Zealand Pharmacoviligance Centre’s Centre for Adverse Reactions Monitoring (CARM) at https://nzphvc.otago.ac.nz/consumer-reporting/.